The following is a guest post from Mentor, an ISAPS Platinum Global Sponsor.
![]()
What the 2020 – 2024 ASPS data tells us about the next generation of patients
The following article highlights data from the U.S., but the challenges and opportunities it explores prompts a broader conversation about how patient needs and surgical practices are evolving worldwide. What are your Gen Z patients asking for in consults?
Gen Z isn’t just holding steady in breast augmentation, they’re reshaping the mindset.

In 2020, patients aged 20-29 accounted for over 52,000 breast augmentations.1
By 2024, that number is still holding steady.2
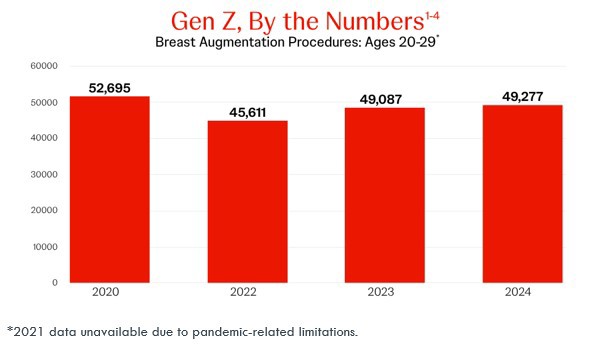
This consistency suggests early engagement, and long-term relevance.2
At J&J Medtech, we believe that education, safety, and personalization should meet every
generation where they are, and where they’re headed.

As younger patients continue to engage earlier2, our role is to support informed decisions,
grounded in data and delivered with care.
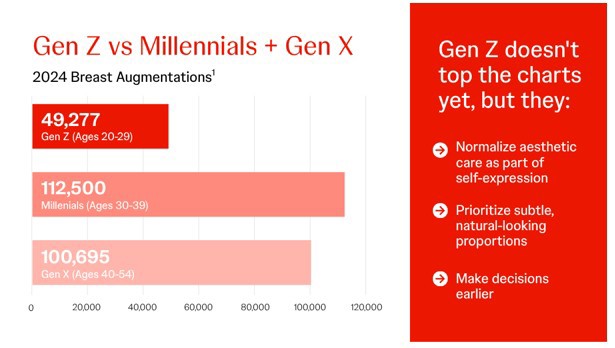
What Surgeons can take from the Data1-7
- Gen Z (20-29) has maintained steady breast augmentation volumes since 2020
- They're choosing earlier than older generations
- Their priorities may differ and deserve deeper understanding


The sale and distribution of Mentor Breast Implant Devices are restricted to users and/or user facilities that provide information to patients about the risks and benefits of the device prior to its use in the form and manner specified in approved labeling to be provided by Mentor Worldwide LLC.
Important information: Prior to use, refer to the instructions for use supplied with this device for indications, contraindications, side effects, warnings and precautions.
Caution: US law restricts this device to sale by or on the order of a physician.
Important Safety Information:
The MENTOR™ Collection of Breast Implants are indicated for breast augmentation - in women who are at least 22 years old for MENTOR™ MemoryGel™ Breast Implants or MENTOR™ MemoryShape™ Breast Implants, and at least 18 years old for MENTOR™ Saline Breast Implants. Breast implant surgery should not be performed in women:
- With active infection anywhere in their body
- With existing cancer or pre-cancer of their breast who have not received adequate treatment for those conditions
- Who are currently pregnant or nursing
Safety and effectiveness have not been established in patients with autoimmune diseases (for example lupus and scleroderma), a weakened immune system, conditions that interfere with wound healing and blood clotting, or reduced blood supply to breast tissue. Patients with a diagnosis of depression, or other mental health disorders, should wait until resolution or stabilization of these conditions prior to undergoing breast implantation surgery.
Breast implants are not lifetime devices and breast implantation may not be a one-time surgery. There are risks associated with breast implant surgery. The chance of developing complications increases over time. You may need additional unplanned surgeries on your breasts because of complications or unacceptable cosmetic outcomes. Many of the changes to your breast following implantation are irreversible (cannot be undone) and breast implants may affect your ability to breastfeed, either by reducing or eliminating milk production.
The most common complications for breast augmentation with MemoryGel™ Implants include any reoperation, capsular contracture, nipple sensation changes, and implant removal with or without replacement. The most common complications with MemoryShape™ Implants for breast augmentation include reoperation for any reason, implant removal with or without replacement, and ptosis. A lower risk of complication is rupture. The health consequences of a ruptured silicone gel breast implant have not been fully established. MRI screenings are recommended three years after initial implant surgery and then every two years after to detect silent rupture. Breast implants are also associated with the risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), an uncommon type of lymphoma. An individual's risk of developing BIA-ALCL with MENTOR™ Breast Implants is low based on the incidence of worldwide cases.
Detailed information regarding the risks and benefits associated with MENTOR™ Breast Implants is provided in several educational brochures. For MemoryGel™ Implants: Important Information for Augmentation Patients about MENTOR™ MemoryGel™ Breast Implants. For MemoryShape™ Implants: Patient Educational Brochure – Breast Augmentation with MENTOR™ MemoryShape™ Breast Implants and Quick Facts about Breast Augmentation & Reconstruction with MENTOR™ MemoryShape™ Breast Implants. For MENTOR™ Saline-filled Implants: Saline-Filled Breast Implants: Making an Informed Decision. These brochures are available from your surgeon or visit www.mentorwwllc.com. It is important that you read and understand these brochures when considering MENTOR™ Breast Implants.
C_ US_MNT_AMNT_ 404798. EN
© Johnson & Johnson and its affiliates 2025
ISAPS Global Sponsors
